KRONOS trial6
Double blind; No requirement for a 1-year exacerbation history
74% of patients in this trial did not have any history of moderate or severe exacerbation in the year prior to screening
- Length: 24 weeks
- Population: Aged 40–80 years
TRIXEO
ICS/LAMA/LABA pMDI
320/18/9.6 μg BID
(n=640)
LAMA/LABA pMDI
18/9.6 μg BID
(n=627)
- Change from baseline in morning predose trough FEV1 over 24 weeks
- FEV1 AUC0-4 over 24 weeks
ICS/LABA pMDI
320/9.6 μg BID
(n=316)
Open-Label ICS/LABA DPI
400/12 μg BID
(n=319)
Key secondary endpoints:
- Rate of moderate or severe COPD exacerbations
- Change from baseline in morning predose trough FEV1
TRIXEO: SUSTAINED IMPROVEMENTS
IN LUNG FUNCTION1,6
TRIXEO significantly improved morning predose trough FEV1 over 24 weeks vs dual therapies1,6
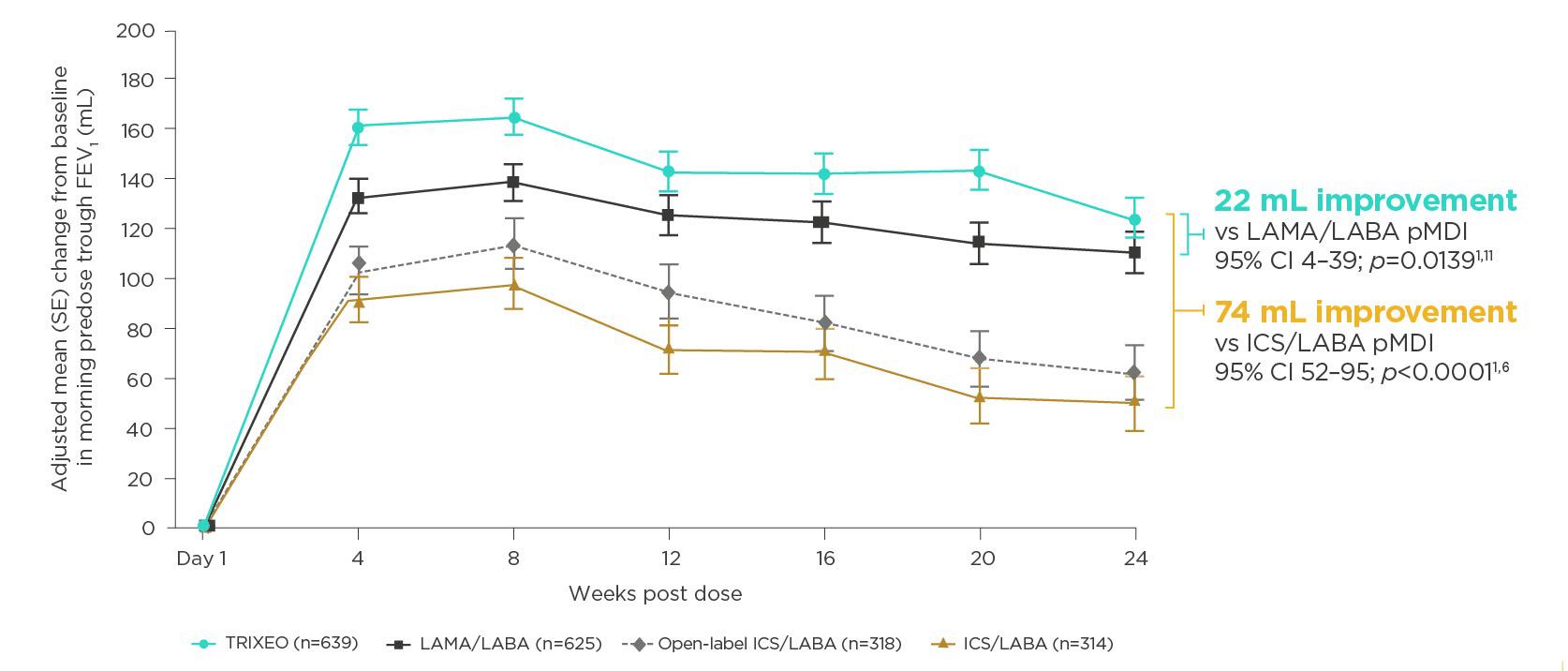
KRONOS was a 24-week trial that compared TRIXEO with GFF pMDI (LAMA/LABA) and BFF pMDI or DPI (ICS/LABA).6
AUC0–4, area under the curve 0–4; BFF, budesonide/formoterol fumarate dihydrate; DPI, dry powder inhaler; FEV1, volume that has been exhaled at the end of the first second of forced expiration; GFF, glycopyrronium/formoterol fumarate dihydrate; ICS, inhaled corticosteroids; ITT, intention-to-treat; LABA, long-acting beta2-agonist; LAMA, long-acting muscarinic antagonist; pMDI, pressurised metered-dose inhaler; SE, standard error.
TRIXEO: SUSTAINED IMPROVEMENTS in FEV1 AUC0–4
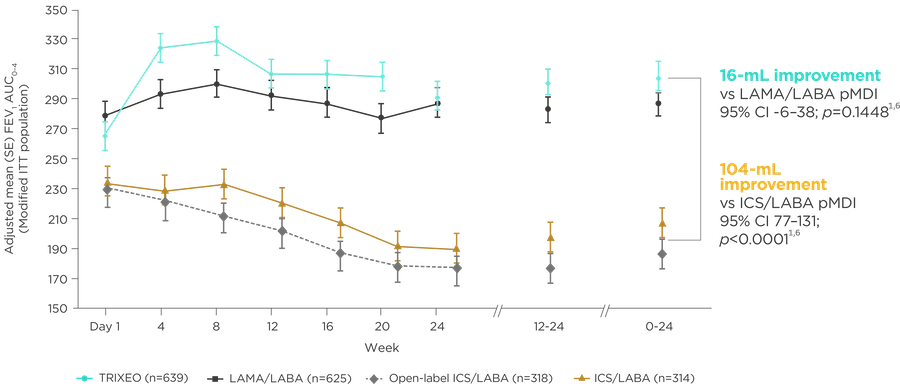
KRONOS was a 24-week trial that compared TRIXEO with GFF pMDI (LAMA/LABA) and BFF pMDI or DPI (ICS/LABA).6
TRIXEO: PROTECTION AGAINST EXACERBATIONS1,6
TRIXEO reduced the rate of moderate or severe exacerbations by 52% vs LAMA/LABA1,6
(secondary endpoint)
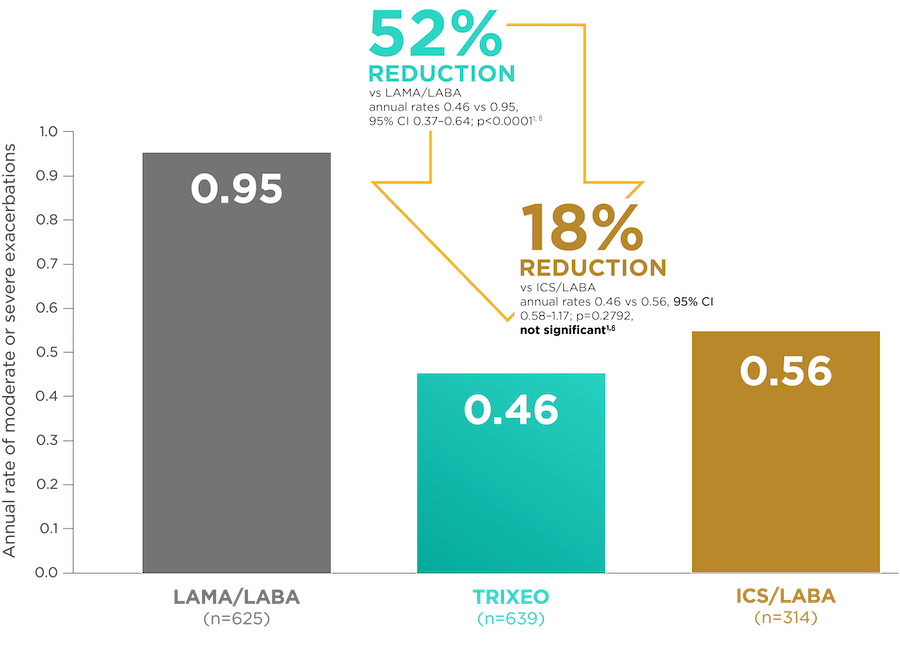
KRONOS was a 24-week trial that compared TRIXEO with GFF pMDI (LAMA/LABA) and BFF pMDI or DPI (ICS/LABA).1
TRIXEO: PROTECTION AGAINST
COPD Hospitalisations3
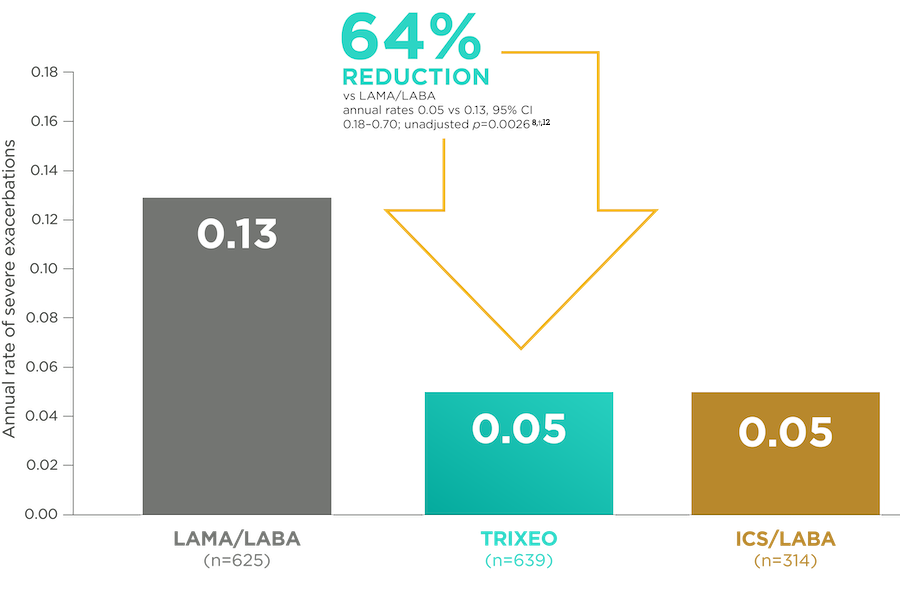
KRONOS was a 24-week trial that compared TRIXEO with GFF pMDI (LAMA/LABA) and BFF pMDI or DPI (ICS/LABA).1
Adverse events that occurred in ≥2% of patients in any group (safety population) in the KRONOS study6
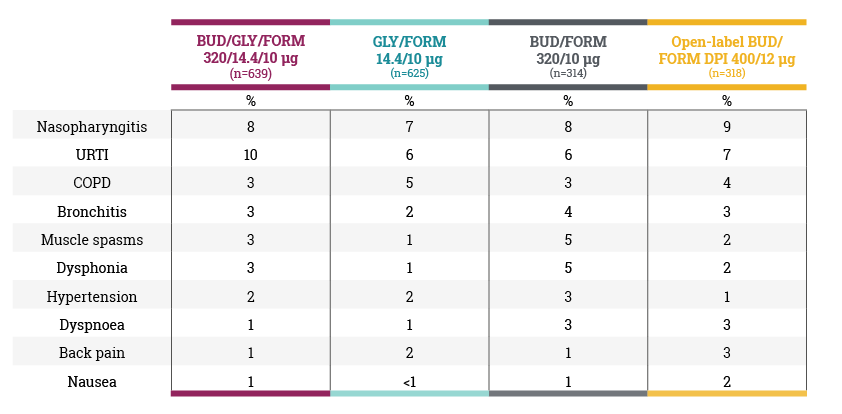
In the clinical trial programme for TRIXEO, LAMA/LABA refers to glycopyrronium/ formoterol fumarate and ICS/LABA refers to budesonide/formoterol fumarate.
BFF, budesonide/formoterol fumarate dihydrate; COPD, chronic obstructive pulmonary disease; GFF, glycopyrronium/formoterol fumarate dihydrate; ICS, inhaled corticosteroids; LABA, long-acting beta2-agonist; LAMA, long-acting muscarinic antagonist; OCS, oral corticosteroids; pMDI, pressurised metered-dose inhaler DPI, dry powder inhaler; FEV1, volume that has been exhaled at the end of the first second of forced expiration;
