THE RISK OF PNEUMONIA ASSOCIATED WITH TRIXEO
IS LOW RELATIVE TO ITS BENEFITS FOR EXACERBATION PREVENTION10
A post hoc analysis (n=8509) quantified the exacerbation benefits relative
to pneumonia risk (expressed as NNT and NNH, respectively) in ETHOS10
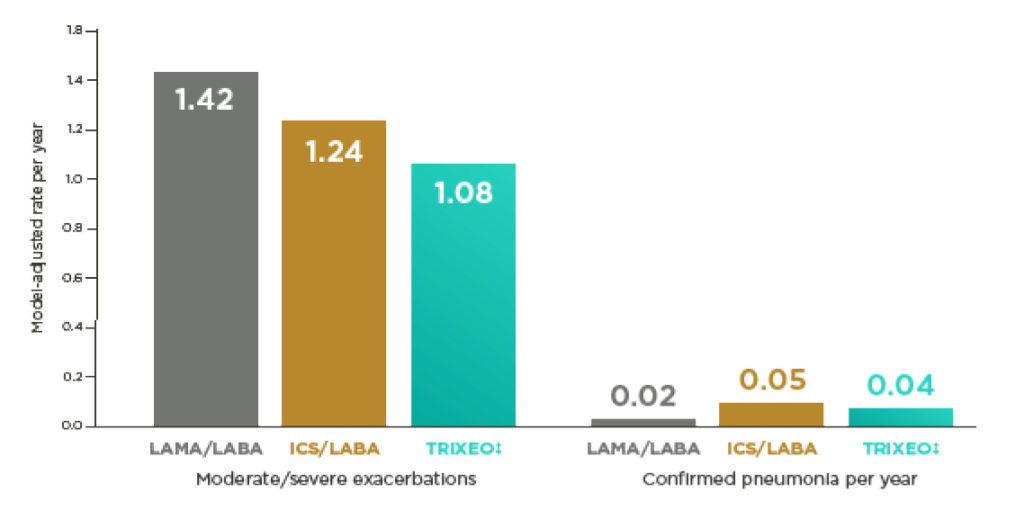
The NNT* for 1 year with TRIXEO to prevent 1 moderate or severe COPD exacerbation was 3 (95% CI 3–5) and 7 (95% CI 4–18) vs LAMA/LABA or ICS/LABA, respectively10

The NNH* for there to be 1 extra case of confirmed pneumonia after 1 year of treatment with TRIXEO was 58 (95% CI 29–152) vs LAMA/LABA vs ICS/LABA10†
CI, confidence interval; COPD, chronic obstructive pulmonary disease; ICS, inhaled corticosteroids; LABA, long-acting beta2-agonist;
LAMA, long-acting muscarinic antagonist; pMDI, pressurised metered-dose inhaler.
*NNT is the number needed to treat for 1 year to prevent 1 moderate/severe COPD exacerbation.
NNH is the number needed to treat for 1 year for there to be 1 extra case of confirmed pneumonia.
NNT and NNH calculated using 1/(rateBGF pMDI – ratecomparator). 10
†NNH for TRIXEO vs ICS/LABA not shown as the rate was lower for TRIXEO than ICS/LABA.17
‡The strength of TRIXEO referred to here is 320/18/9.6 μg
