ETHOS Trial2
Double blind; ≥1 moderate or severe COPD exacerbation(s) in year prior to screening
The ETHOS trial (N=8588): All patients had ≥1 moderate or severe COPD exacerbation(s) in year prior to screening
- Length: 52 weeks
- Population: Aged 40–80 years
TRIXEO
ICS/LAMA/LABA pMDI
320/18/9.6 μg BID
(n=2157)
ICS/LAMA/LABA pMDI
160/18/9.6 μg BID (n=2137)
- Annual rate of moderate or severe COPD exacerbations (as estimated over 52 weeks)*
LAMA/LABA pMDI
18/9.6 μg BID
(n=2143)
ICS/LABA pMDI
320/9.6 μg BID
(n=2151)
Key secondary endpoints:
- Annual rate of severe COPD exacerbations
- Change from baseline in SGRQ total score over 24 weeks
- Time to death from any cause
TRIXEO: PROTECTION AGAINST
MODERATE OR SEVERE EXACERBATIONS2
vs LAMA/LABA and ICS/LABA2
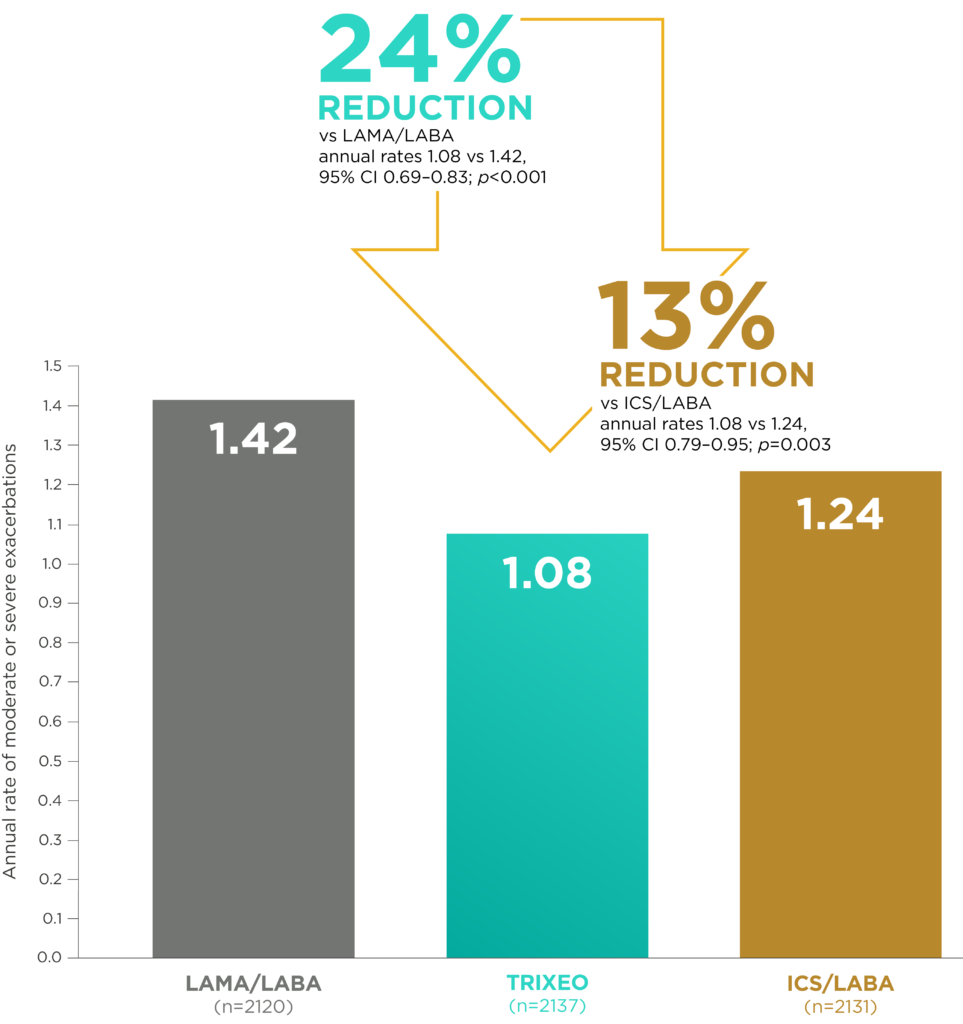
The NNT* for 1 year with TRIXEO to prevent 1 moderate or severe COPD exacerbation was 3 (95% CI 3–5) and 7 (95% CI 4–18) vs LAMA/LABA or ICS/LABA, respectively10
DEFINITION OF EXACERBATIONS
Moderate exacerbations were defined as events requiring the use of OCS/systemic corticosteroids and/or antibiotics for at least 3 days. Severe exacerbations were defined as events resulting in inpatient COPD-related hospitalisation or COPD-related death.2,3
*NNT is the number needed to treat for 1 year to prevent 1 moderate/severe COPD exacerbation. NNH calculated using 1/(rateBGF pMDI – ratecomparator)10
TRIXEO: PROTECTION AGAINST
COPD HOSPITALISATIONS2*
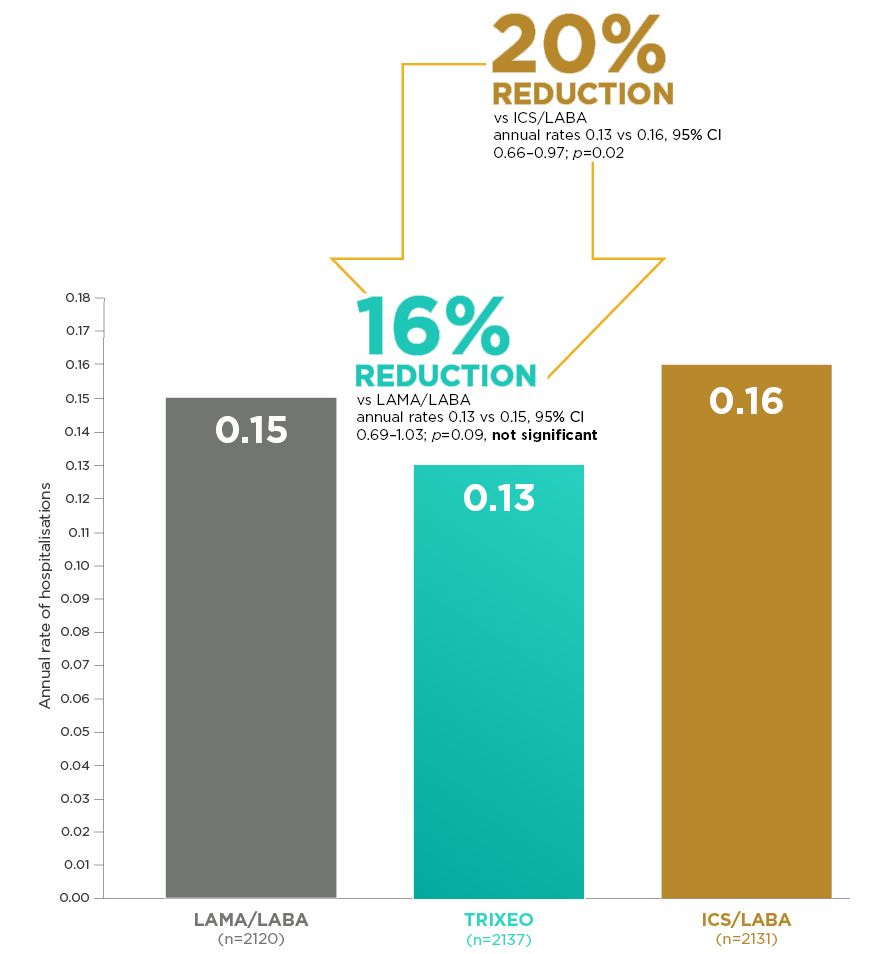
ETHOS was a 52-week trial that compared TRIXEO with GFF pMDI (LAMA/LABA) and BFF pMDI (ICS/LABA).1
*Hospitalisations defined as exacerbation events resulting in inpatient COPD-related hospitalisation or COPD-related death2
TRIXEO: REDUCES SYMPTOMS
OF COPD AND IMPROVES QUALITY OF LIFE4,5
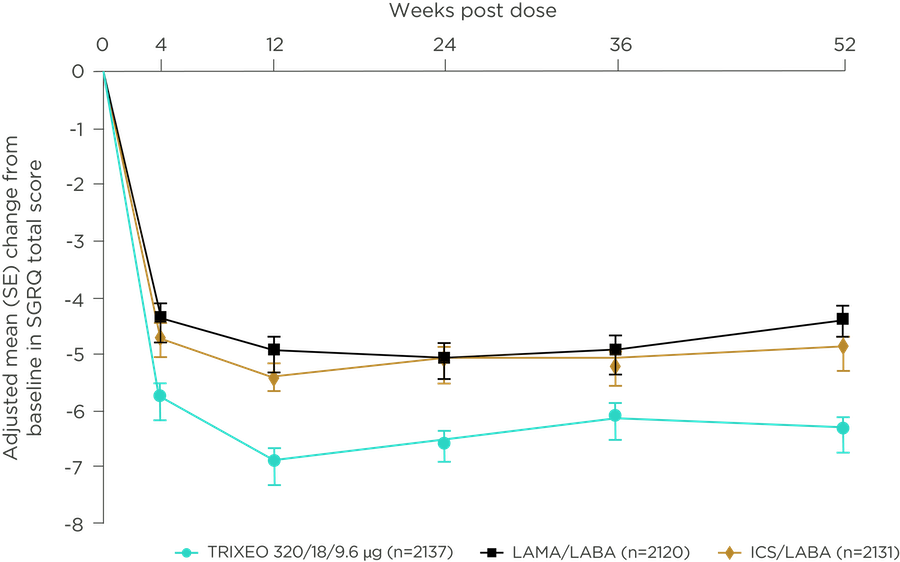
Adapted from Rabe KF et al. N Engl J Med. 2020
Improvement in SGRQ
over 24 weeks
–1.62 difference
vs LAMA/LABA
–1.38 difference
vs ICS/LABA
ETHOS was a 52-week trial that compared TRIXEO with GFF pMDI (LAMA/LABA) and BFF pMDI (ICS/LABA).1 Total scores on the SGRQ range from 0 to 100, with lower scores indicating better health-related quality of life; the minimum clinically important difference is 4 units.5
Adverse events that occurred in ≥3% of patients in any group (safety population) in the ETHOS study5
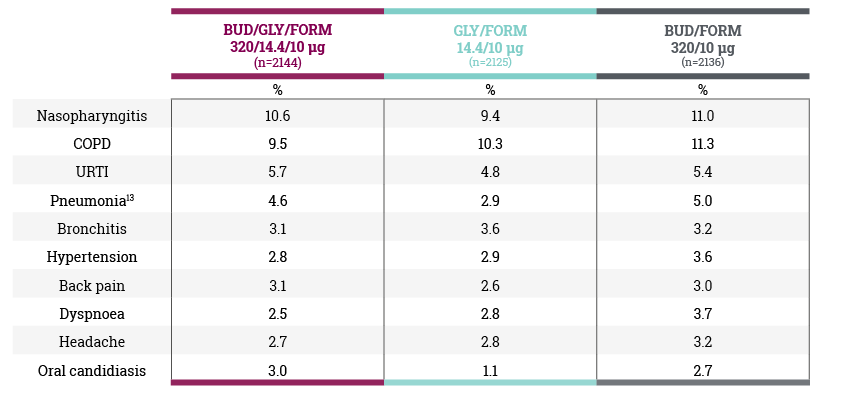
In the clinical trial programme for TRIXEO, LAMA/LABA refers to glycopyrronium/ formoterol fumarate and ICS/LABA refers to budesonide/formoterol fumarate.
BFF, budesonide/formoterol fumarate dihydrate; COPD, chronic obstructive pulmonary disease; GFF, glycopyrronium/formoterol fumarate dihydrate; ICS, inhaled corticosteroids; LABA, long-acting beta2-agonist; LAMA, long-acting muscarinic antagonist; OCS, oral corticosteroids; pMDI, pressurised metered-dose inhaler
